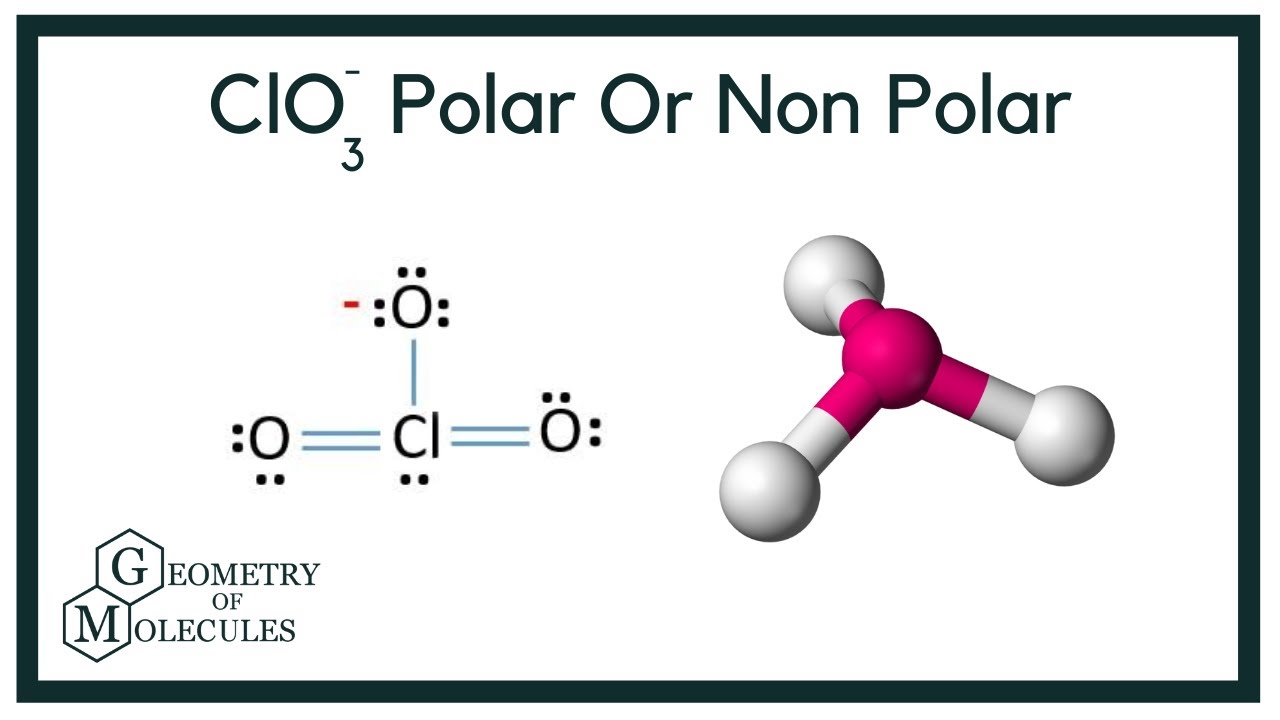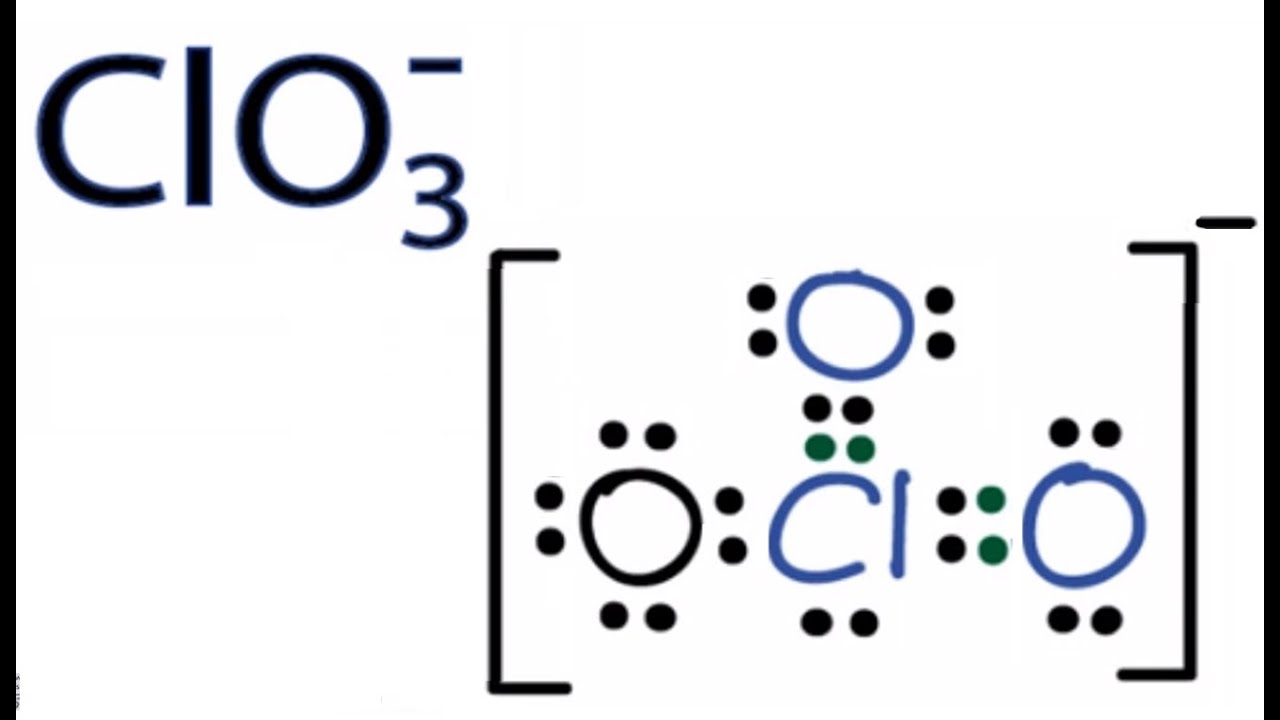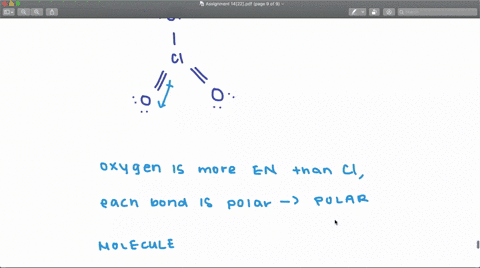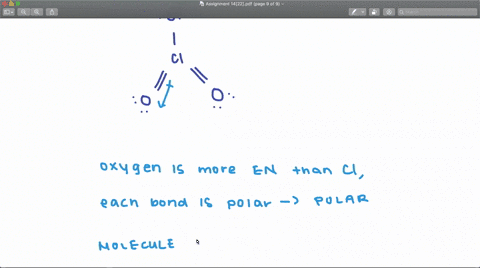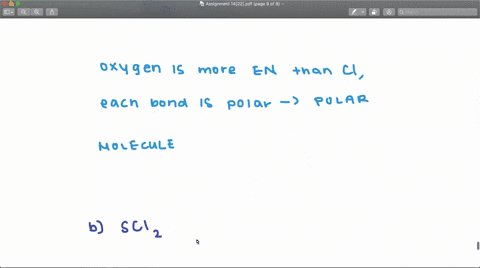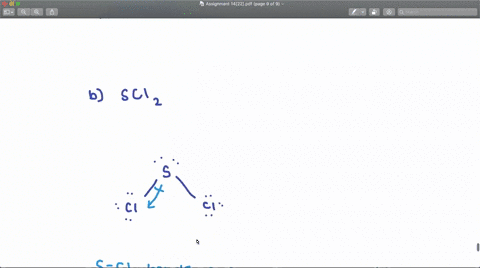
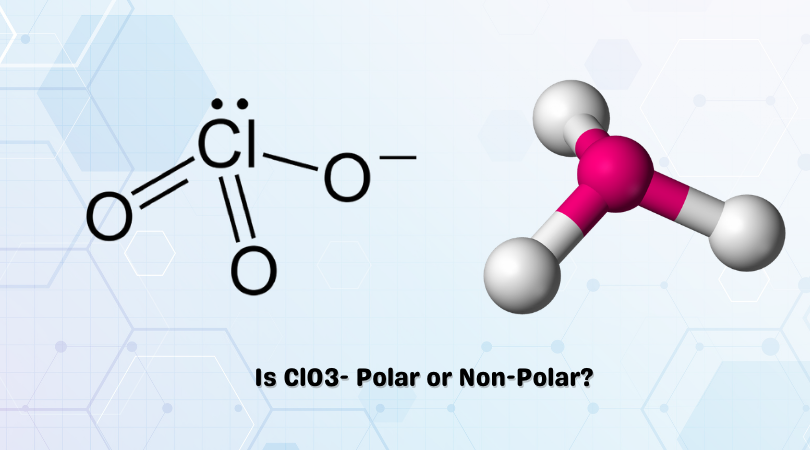
SOLVED: Determine whether each molecule or ion is polar or nonpolar. a. ClO3 - b. SCl2 c. SCl4 d. BrCl5

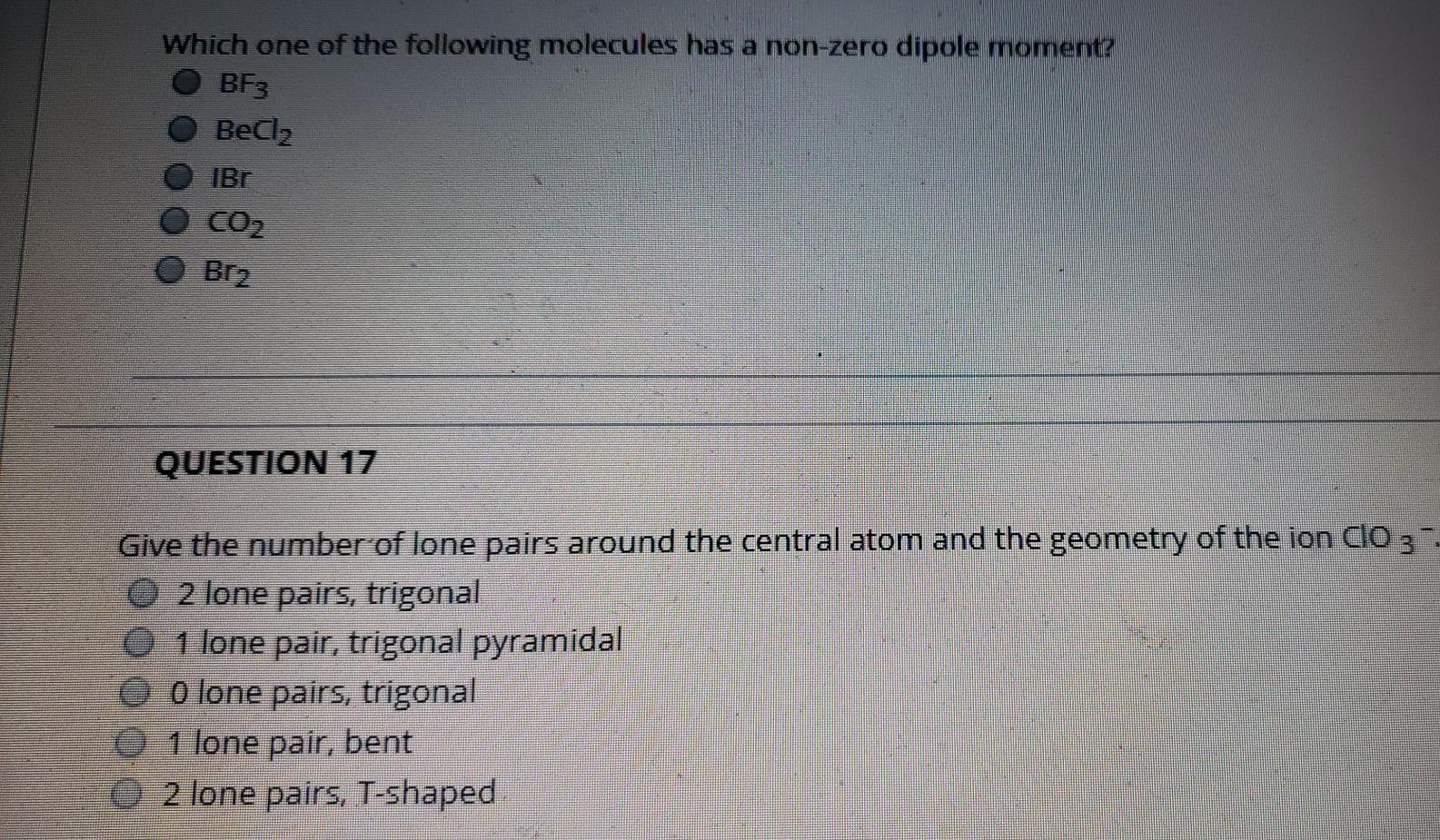
SOLVED:Draw Lewis structures and give the other information requested for the following molecules: (a) BF3 Shape: planar or nonplanar? (b) ClO3^-. Shape: planar or nonplanar? (c) H2 O. Show the direction of
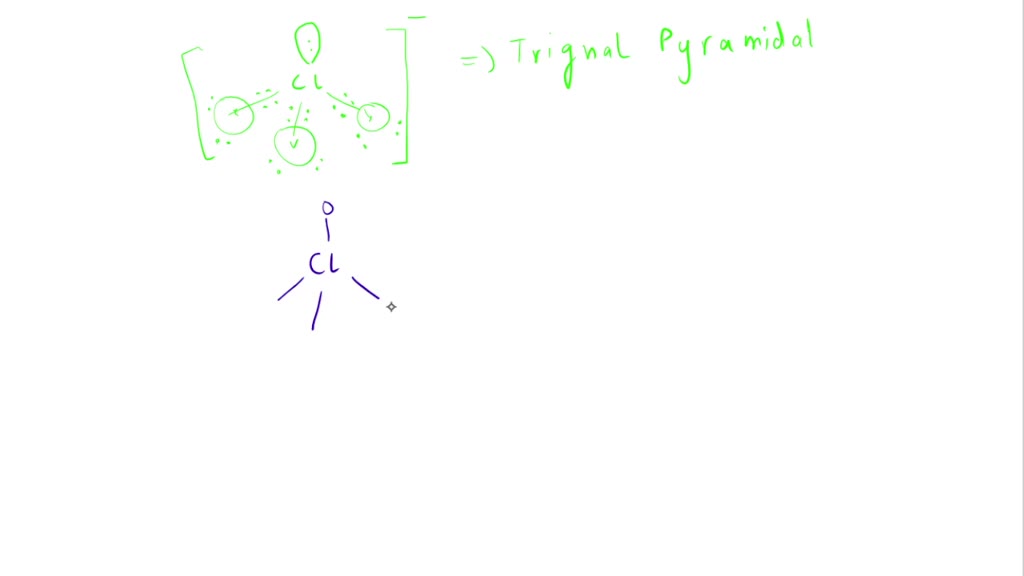
SOLVED: Draw the Lewis structures of ClO3- and ClO4- (Cl is the central atom in both cases). Explain why ClO3- has an overall dipole moment and why ClO4- doesn't.

![Dipole Moment |Chemical Bonding| in urdu/hindi [Easy & Simple] - YouTube Dipole Moment |Chemical Bonding| in urdu/hindi [Easy & Simple] - YouTube](https://i.ytimg.com/vi/SI5LkAJE-ec/hqdefault.jpg)