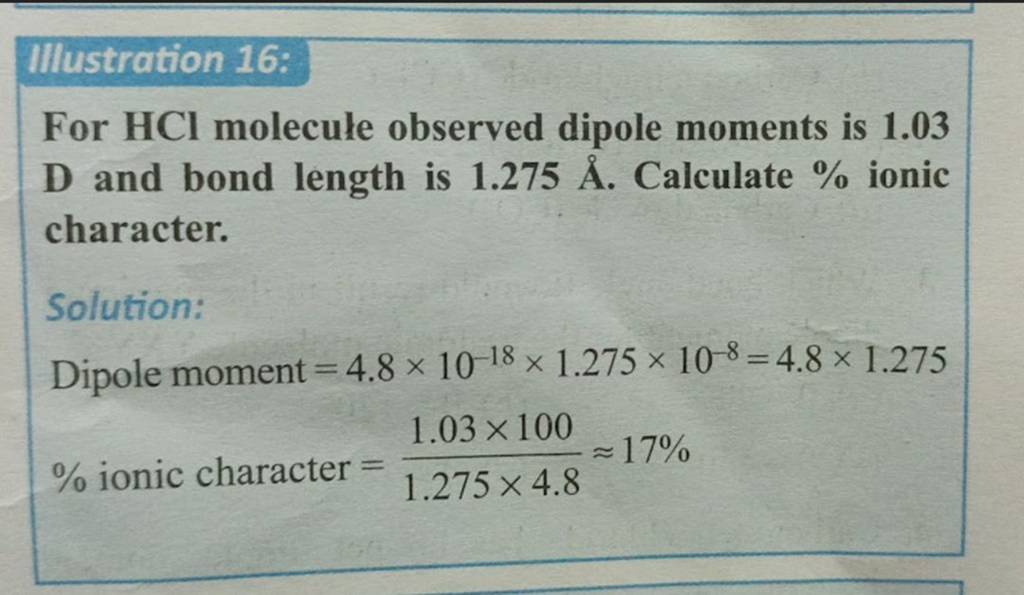
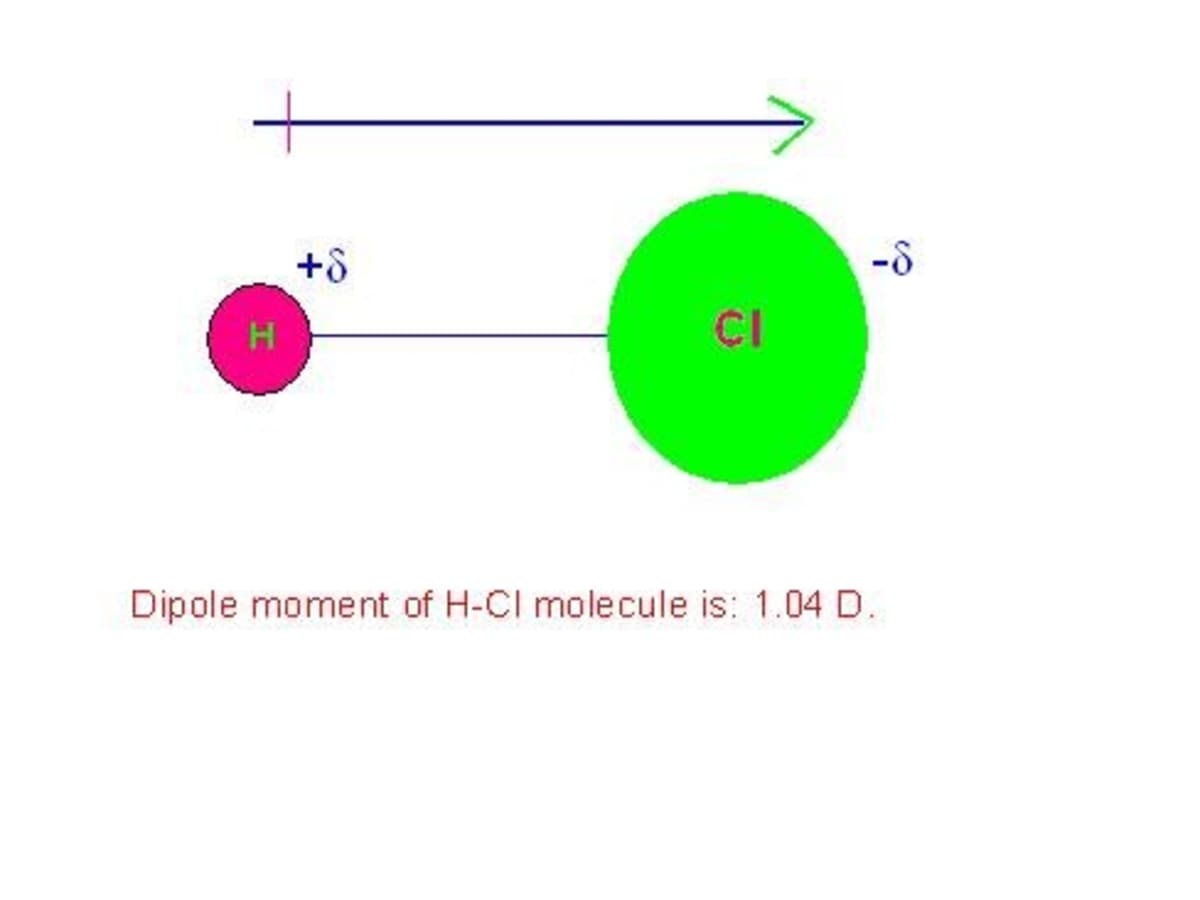
the observed dipole moment of hcl is 1.03D bond length is 1.275A then the precentage of ionic character is
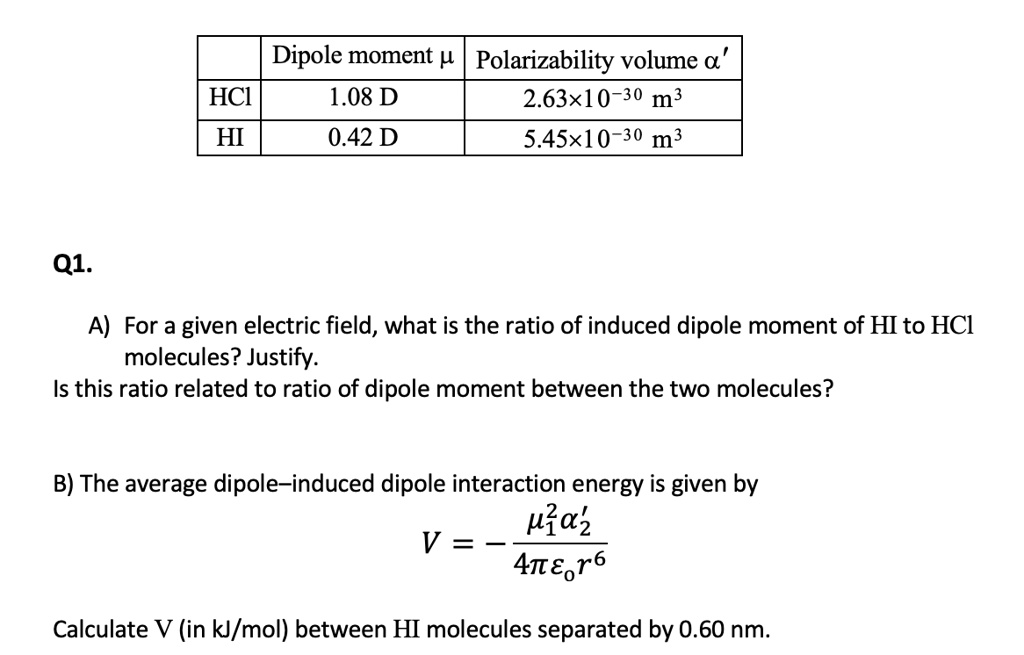
SOLVED: Dipole moment (μ) Polarizability volume " HCl 1.08 D 2.63x10^-30 m^3 HI 0.42 D 5.45x10^-30 m^3 Q1. A) For a given electric field, what is the ratio of the induced dipole
Why is it so that the dipole moment of HI < HBr< HCl<HF, where HI is lowest and HF is highest? - Quora

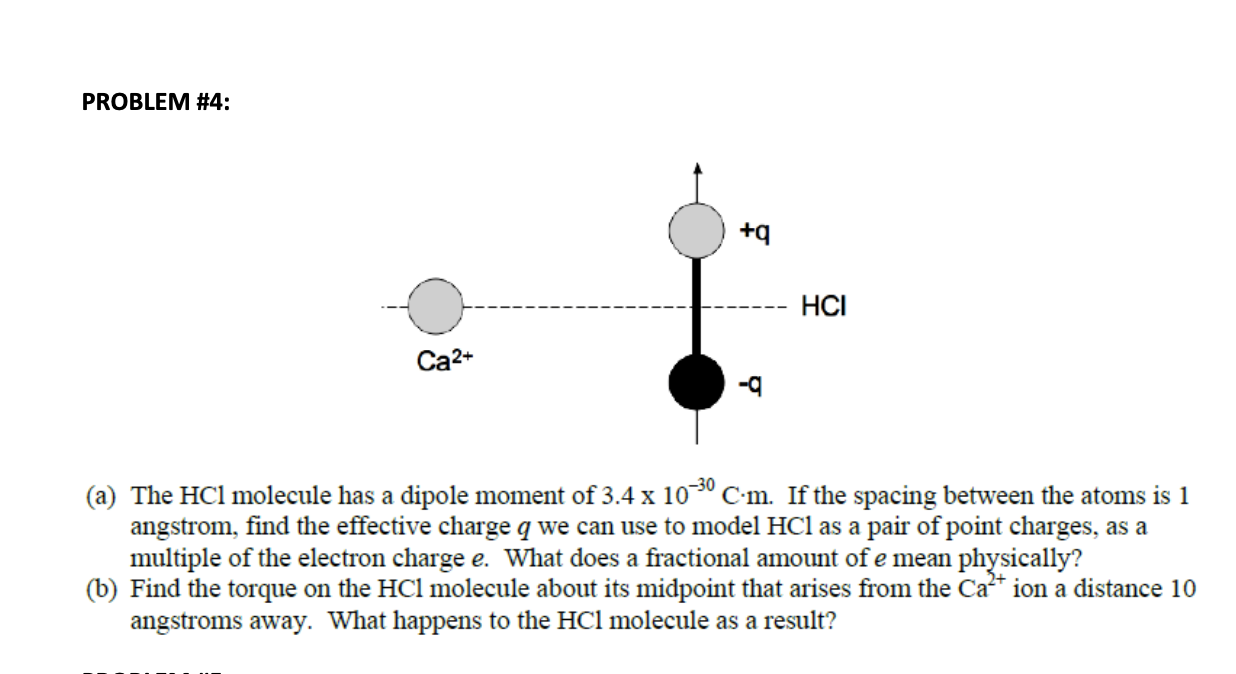
An HCl molecule has a dipole moment of `3.4xx 10(-30) Cm` . Assuming that equal and opposite cha... - YouTube

TAMLIQ BJ VILIVM LIQUI MV LIQ The electric dipole moment of an HCL atom is 3.4 x 10-30 cm. The charges on both atoms are unlike and of same magnitude. Magnitude of

20., The observed dipole moment of HCl is 1.03 D.Bond length is 1.275 A then the percentage ofionic - Brainly.in

The experimental dipole moment of HCl is 1.03D and its bond length (distance) is 1.27 Å..... - YouTube
In HCl molecule, expected value of dipole moment is 6.12 D but experimental value is 1.03 D. Then, the percentage ionic character will be Options: a) 16.83 b) 15.14 c) 6.02 d) 18.90



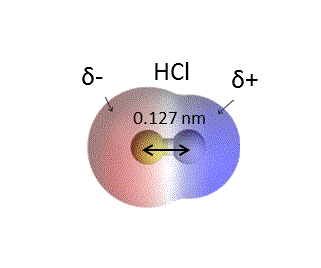





![[Bengali] Calculate the % ionic character in HCI molecule. Given bond [Bengali] Calculate the % ionic character in HCI molecule. Given bond](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/8903493.webp)